Myasthenia Gravis Resource Center
NEW: MuSK Myasthenia Gravis
Syncope as a Presenting Symptom of MuSK-Associated Myasthenia Gravis
The cases we presented demonstrated diverse clinical presentations and outcomes, highlighting the necessity for attentive monitoring and prompt management of such complications. Clinicians must be aware of risk factors like non-O blood types and should consider alternative treatment approaches or dosage modifications when prescribing IVIG to patients with NMOSD or other CNS demyelination disorders to mitigate the risk of hemolytic complications.
October 2024
Read moreComparison of Fixed and Live Cell-Based Assay for the Detection of AChR and MuSK Antibodies in Myasthenia Gravis
Live cell-based assay (CBA) can detect acetylcholine receptors (AChRs) or muscle-specific tyrosine kinase (MuSK) antibodies (Abs) in a proportion of patients with radioimmunoassay (RIA)-double seronegative myasthenia gravis (dSN-MG). A commercial fixed CBA for AChR and MuSK Abs has recently become available; however, comparative studies on fixed and live CBAs are lacking. In this study, we compared the performance of fixed and live CBAs in patients with RIA-dSN MG and assessed their sensitivity in RIA-positive MG samples and their specificity.
Nov 22, 2024
Read moreMuSK Antibody Positive Myasthenia Gravis Mimicking as Myositis
We report a 40-year-old female patient, who visited our hospital with presenting complaints of breathlessness and generalized myalgia and subsequently developed fatigable weakness during hospitalization.
September 2024
Read moreSerological Markers of Clinical Improvement in MuSK Myasthenia Gravis
In this retrospective longitudinal study, we aimed at exploring the role of (a) MuSK-immunoglobulin G (IgG) levels, (b) predominant MuSK-IgG subclasses, and (c) antibody affinity as candidate biomarkers of severity and outcomes in MuSK-MG, using and comparing different antibody testing techniques.
September 2024
Read moreThyroid eye disease and ocular myasthenia gravis
An overview of two ocular diseases, which significantly impact quality of life: thyroid eye disease (TED) and ocular myasthenia gravis (OMG). Additionally, we describe the clinical challenge when they occur simultaneously. We will describe the pathophysiology of both conditions, the currently available diagnostic tools, and the therapies available.
Read moreMulticenter Validation of the Ocular Myasthenia Gravis Rating Scale Questionnaire
Ocular myasthenia gravis (OMG) causes disabling ocular symptoms of ptosis and diplopia, but a validated disease-specific patient-reported outcome measure (PROM) has not been reported. We sought to validate a novel PROM for OMG, OMG Rating Scale Questionnaire (OMGRate-q), as a measure of visual functioning to support patient-centered care.
Read morePeribulbar Corticosteroids for Ocular Myasthenia Gravis
Ocular myasthenia gravis is treated predominantly by oral medications, with the potential for systemic adverse effects. Successful treatment has been achieved using peribulbar dexamethasone. We assessed the effect of peribulbar dexamethasone or triamcinolone (40-mg Triesence), a longer-acting corticosteroid, targeting the peribulbar area as opposed to directly injecting the affected extraocular muscle. This more convenient and secure approach holds the potential for straightforward integration within clinical environments.
Read moreOphthalmologic clinical features of ocular myasthenia gravis
To investigate the clinical features of ocular myasthenia gravis (OMG) in ophthalmology. A total of 28 patients with ptosis or diplopia who were followed for at least 6 months between March 2016 and February 2022 were included in this study. The clinical symptoms of the patients and test results were analyzed.
Read moreEffect of Immunosuppression in Risk of Developing Generalized Symptoms in Ocular Myasthenia Gravis
Early use of immunosuppression has been suggested to prevent generalization of ocular myasthenia gravis (OMG), but high-quality evidence is limited in this regard. We examined whether treatment with prednisone and other immunosuppressants reduce the risk of generalization in OMG.
Read morePearls & Oy-sters: Ocular Myasthenia Gravis
Myasthenia gravis (MG) has been described as a great mimicker of other neurologic and ocular motility disorders, including centrally mediated ophthalmoplegia. For example, ocular myasthenia gravis (ocular MG) may cause impaired binocular visual acuity for near vision due to reduced accommodation or for distance vision due to accommodative excess.
Read moreAn unusual initiation of an ocular form of MuSK-positive myasthenia gravis after magnesium administration: a rare case report
A 26-year-old pregnant patient was referred to the Neurology Department after experiencing tongue enlargement. A neuro-ophthalmic assessment revealed ptosis with lateral diplopia in the right eye, bulbar palsy, facial weakness, weakness in the palate and pharyngeal reflex, dizziness, and hearing loss in her right ear.
Read moreTest–Retest Reliability of Repetitive Ocular Vestibular Evoked Myogenic Potentials in Myasthenia Gravis Patients and Healthy Control Subjects
Repetitive ocular vestibular evoked myogenic potentials (ROVEMP) are a novel diagnostic test to quantify neuromuscular transmission deficits in extraocular muscles in myasthenia gravis. We aimed to investigate the test–retest reliability of the ROVEMP and the effect of amplitude and age.
Read moreNew!
Neurology® Video Journal Club: ALS, Myasthenia Gravis, & Neuromuscular Collection
Learn from experts as they discuss recent Neurology journal articles and hot topics in the field of neurology,
Visit Now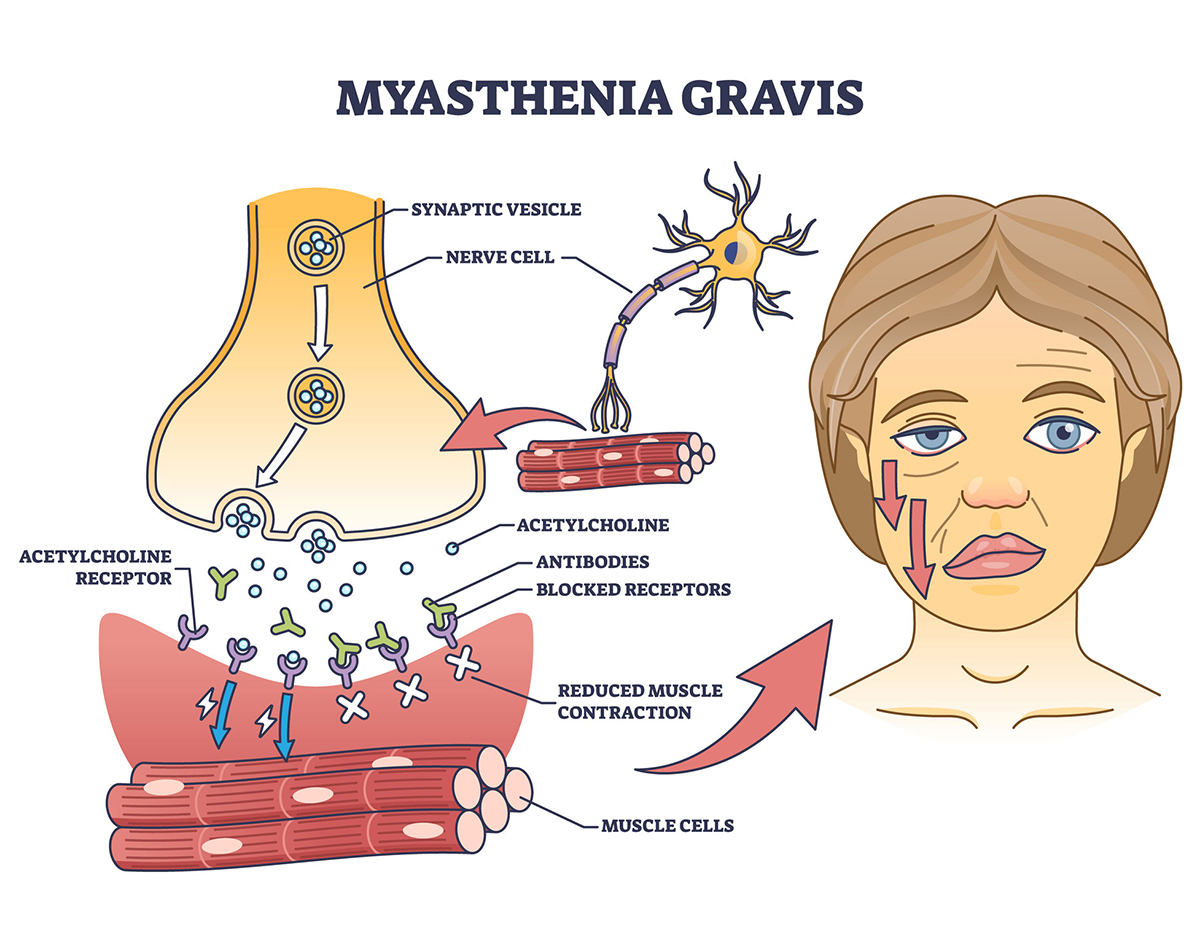
Therapeutic Responses to Efgartigimod for Generalized Myasthenia Gravis in Japan
Efgartigimod, which has been well tolerated and efficacious in individuals with generalized myasthenia gravis (MG), is available in Japan not only for the treatment of anti–acetylcholine receptor–positive (AChR+) but also anti–muscle-specific receptor tyrosine kinase (MuSK+) and seronegative generalized MG. We report details of the use of efgartigimod for generalized MG in clinical practice in Japan.
July 2024
Read more
The nurse’s guide to myasthenia gravis
Myasthenia gravis (MG) is an autoimmune disease that causes a defect in the postsynaptic membrane in the neuromuscular junction (NMJ), resulting in excessive fatigue and fluctuating muscle weakness.1 The affected muscles may include those that control eyelid motion, facial expression, chewing, swallowing, speaking, and the extremities. Weakness may worsen with activity and improve with rest. Symptoms may be mild but can become life-threatening if the disease affects respiratory muscles because this can result in respiratory failure.1 This article presents the pathogenesis, signs, symptoms, and management of patients with MG.
July 2024
Read more
Functional Signature of LRP4 Antibodies in Myasthenia Gravis
Antibodies (Abs) specific for the low‐density lipoprotein receptor-related protein 4 (LRP4) occur in up to 5% of patients with myasthenia gravis (MG). The objective of this study was to profile LRP4-Ab effector actions.
July 2024
Read more
Hospitalizations and Mortality From Myasthenia Gravis (article!)
Myasthenia gravis (MG) is a rare neuromuscular disorder where IgG antibodies damage the communication between nerves and muscles, leading to muscle weakness that can be severe and have a significant impact on patients’ lives.
February 2024
Read more
Hospitalizations and Mortality From Myasthenia Gravis
Drs. Derek Stitt and Ali A. Habib discuss trends in hospital admissions and in-hospital mortality for adult patients with myasthenia gravis.
Jan 2024
Read more
Exploring Factors That Prolong the Diagnosis of Myasthenia Gravis
Myasthenia gravis (MG) is a condition with significant phenotypic variability, posing a diagnostic challenge to many clinicians worldwide. Prolonged diagnosis can lead to reduced remission rates and morbidity. This study aimed to identify factors leading to a longer time to diagnosis in MG that could be addressed in future to optimize diagnosis time.
March 2024
Read more
Efgartigimod in generalized myasthenia gravis: A real-life experience at a national reference center
Inhibition of the neonatal Fc receptor (FcRn) for IgG is a promising new therapeutic strategy for antibody-mediated disorders. We report our real-life experience with efgartigimod (EFG) in 19 patients with generalized myasthenia gravis (gMG) along a clinical follow-up of 14 months.
December 2023
Read moreRelated Articles

Pregnancy planning may impact maternal and neonatal outcomes in people with myasthenia gravis
Myasthenia Gravis (MG) is an acquired autoimmune condition commonly diagnosed in young people of reproductive age resulting in neuromuscular junction dysfunction. The course of MG during pregnancy and its impact on maternal and neonatal outcomes is vary in the literature.
December 2023
Read more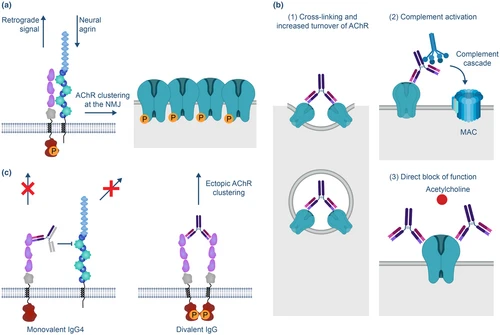
Refocusing generalized myasthenia gravis: Patient burden, disease profiles, and the role of evolving therapy
Generalized myasthenia gravis (gMG) continues to present significant challenges for clinical management due to an unpredictable disease course, frequent disease fluctuations, and varying response to therapy. The recent availability of new pharmacologic therapies presents a valuable opportunity to reevaluate how this disease is classified, assessed, and managed and identify new ways to improve the clinical care of patients with gMG.
December 2023
Read more
Diagnosis and evaluation of elderly-onset myasthenia gravis: A case report
Patients with elderly-onset myasthenia gravis can have a good prognosis with appropriate diagnosis and response, although it is difficult to differentiate between exacerbations of myasthenia gravis in elderly patients and age-related changes. Therefore, it is important for physicians to understand the clinical characteristics and safe assessment methods for patients with elderly-onset myasthenia gravis.
January 2024
Read more
Facial-onset sensory and motor neuronopathy with myasthenia gravis: A case report
Facial-onset sensory and motor neuronopathy (FOSMN) is a greatly rare disease, so far, autopsy evidence that is associated with neurodegenerative. Myasthenia gravis (MG) is an antibody-mediated and complement-involved acquired autoimmune disorder of the post-synaptic neuromuscular junction. There have been few reports about if there is related between the 2. In this study, we present the case of a man who was diagnosed as FOSMN with MG in continuity.
November 2023
Read more
Correlation of fatigue on walking ability in myasthenia gravis patients: a cross-sectional study
Myasthenia gravis (MG) is a neuromuscular junction autoimmune disease characterised of intermittent muscle weakness that increases with activity and recovers with rest.
January 2024
Read more
Effect of Light-Intensity Cycle Ergometer Aerobic Exercise on 2-Min Walking Test Distance and Maximum Oxygen Uptake in Myasthenia Gravis Patients: A Randomized Controlled Trial
Fatigue and impaired functional capacity are more likely to be observed with myasthenia gravis (MG). MG prevalence in the Indonesian population is still limited. MG can benefit from participating in aerobic exercise without causing a decline in function, but relatively few exercise training studies have been conducted in this group of patients. This study analyzed how light-intensity cycle ergometer aerobic exercise influences functional and aerobic capacity in MG.
December 2023
Read more
Child Neurology: Common Occurrence of Narcolepsy Type 1 and Myasthenia Gravis
Narcolepsy with cataplexy and myasthenia gravis are both chronic neurologic conditions causing symptoms of muscle weakness, often affecting facial muscles, and have both been attributed to an immune-mediated etiology. We report an adolescent girl diagnosed with both conditions and discuss possible shared mechanisms and the diagnostic challenges presented by her case to inform and aid clinicians managing children and young people with these rare conditions.
December 2024
Read more
A Rare Case of Checkpoint Inhibitor-Induced Myasthenia Gravis
With rising interest in novel immunotherapies for cancer treatment, reporting on significant side effects becomes essential. There are few case reports on checkpoint inhibitor related Myasthenia Gravis. This case aims to provide knowledge on diagnostic and management strategies with patients that present with similar clinical findings to improve future patient care.
Nov 22, 2024
Read more