The clinical development process of biosimilar medicines
This infographic highlights the strictly regulated biosimilar approval process, which assesses biosimilarity on the basis of the totality of evidence.
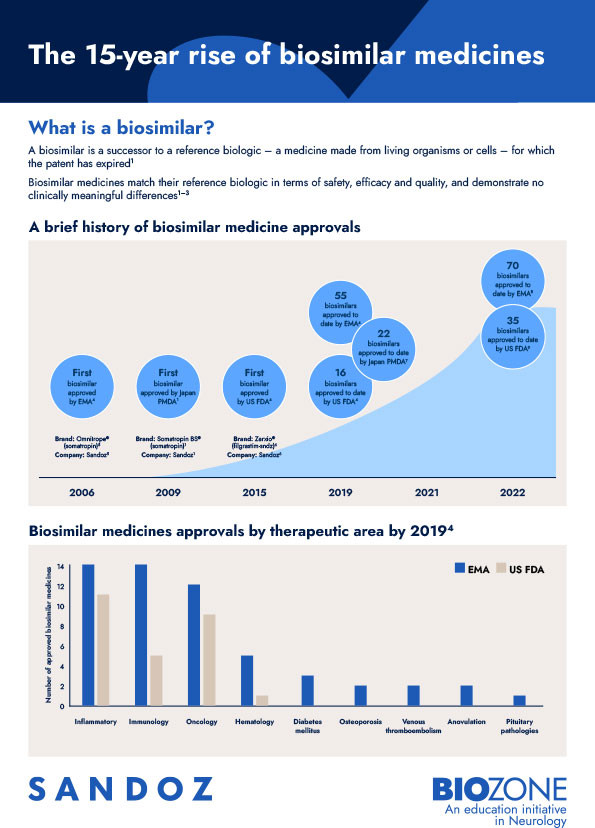
Discover the trajectory of biosimilar approvals since 2006 and their potential future impact on treatment cost and patients’ access to medicines.
Biosimilar medicines match their reference biologic medicines in terms of safety, efficacy and quality, and demonstrate no clinically meaningful differences.1
Experience in other therapy areas, such as rheumatoid arthritis (TNF inhibitors) and oncology (G-CSF), illustrates that biosimilar medicines positively impact patient treatment access and sustainability of care.2,3
References:
1. EMA. Biosimilars in the EU, 2017. Available at: https://www.ema.europa.eu/en/documents/leaflet/ biosimilars-eu-information-guide-healthcare-professionals_en.pdf. [Last Accessed April 2022]
2. Smolen JS, et al. RMD Open 2019;5:e000900.
3. Gascon P, et al. Support Care Cancer 2013;21:2925–2931.
These materials are intended for healthcare professionals only. This page is sponsored by Sandoz.
MLR: 215945
This infographic highlights the strictly regulated biosimilar approval process, which assesses biosimilarity on the basis of the totality of evidence.